Radioactive iodine is potentially very toxic if ingested. Although amounts of activity in this experiment are very low, disposable gloves and eye protection should be worn to avoid direct contact. Under no circumstances should the 125I be used under oxidising conditions when elemental iodine might be formed with a danger of inhalation.
Aim
To understand the technique of radioimmunoassay by performing a chemical ‘mock-up’ of the procedure.
Theory
Radioimmunoassay, or RIA, is an elegant technique for the measurement of very low concentrations (10-6 – 10-12 g cm-3) of physiological, toxicological and therapeutic agents, frequently in body fluids, plasma or serum. The method is based on the reversible reaction between antigens and antibodies. An antigen is any substance which is capable of eliciting an immune response when injected into a host animal. Antigens are usually high molecular weight proteins, polysaccharides and lipoproteins. Low molecular weight substances such as steroid hormones and drugs must usually be attached to a carrier polypeptide or polysaccharide to enhance their ability to stimulate an immune response. Antibodies are immunoglobulins formed in the animal as part of its immune response to an antigen. An antibody usually exhibits a highly specific reactivity towards the antigen that stimulated its formation. Thus, an antibody may be used for the selective detection of a particular antigen in the presence of many other antigenic substances. For example, the antibody produced in a rabbit by the injection of human insulin may subsequently be collected and used for reaction with insulin in human serum.
The reaction between antigen and antibody may be represented as:
AG + AB ↔ AB.AG
The product is an antibody-antigen complex which can usually be separated from excess antigen with comparative ease, by filtration, chromatography or electrophoresis, etc. If the reaction is performed using a radiolabelled antigen, a radiolabelled antibody-antigen complex is obtained.
AG* + AB ↔ AB.AG*
In the assay of an antigen the procedure is to take a known amount of radiolabelled antigen and an amount of antibody which will react with about half of the radiolabelled antigen. Thus, about half of the activity ends up in the antibody-antigen complex, the other half remaining in the solution as labelled antigen.
2AG* + AB ↔ AG* + AB.AG*
The experiment is then repeated with a measured volume (x) of the unknown concentration of (unlabelled) antigen added to the radiolabelled antigen. Now, when the antibody is added the distribution of the radioactivity between antibody-antigen complex and antigen solution will be different and the amount of unlabelled antigen in the sample may be calculated. After separation of AB.AG*, the ratio of activities:
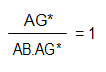
The reaction equation therefore becomes:
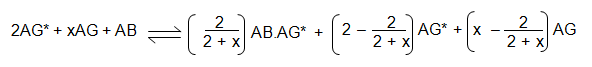
As before, the labelled antigen must be equally split between the AB.AG* and the AG*. This means that:
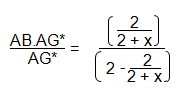
Rearranging this equation gives:
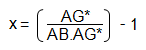
If the concentration of AG*, in say μg cm-3 is conc. (AG*), then concentration of AG may readily be found from:
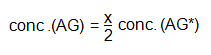
In practice short-lived isotopes are used for the production of labelled antigens for RIA kits, e.g. 131I, t½ ~ 8 days and 125I, t½ 60 days. Thus, the actual concentration of AG* may be difficult to determine accurately, because of the high rate of decay and because of radiation damage to labelled molecules resulting in radiolabelled impurities – which contribute to the activity but probably do not interact with the antibody. Consequently an assay is usually performed by comparing complex activity formed in the presence of the sample antigen concentration with that formed in the presence of known amounts of unlabelled antigen.
Experimental procedure for Introduction to Radioimmunoassay
Questions for Introduction to Radioimmunoassay